Melbourne (Australia) | 31 December 2024
Telix and the Brussels-based Oncidium foundation today announce that results for the NOBLE Registry of TLX599-CDx (99mTc-iPSMA) have been published in the European Journal of Nuclear Medicine and Molecular Imaging (EJNMMI) Reports.
The NOBLE (Nobody Left Behind) Registry (NOBLE) is a global real-world evidence (RWE) study that aims to improve equity of access to state-of-the-art prostate cancer imaging. NOBLE is a collaboration combining the oncology and prostate cancer expertise of the Oncidium foundation as registry sponsor, Telix’s intellectual property, and partnered clinical and operational support. An international network of sites support the registry locally through Principal Investigators (PIs), who are responsible for initiating and conducting research. Investigators collect patient data that will inform the development of TLX599-CDx, an investigational prostate cancer imaging agent that targets PSMA [1] using 99m-technetium (99mTc)-based SPECT[2] imaging.
SPECT cameras are being clinically evaluated in this application because they are more widely available than the PET/CT[3] scanners used for gallium- or fluorine-based PSMA imaging, where access can be limited by socio-economic or geographic factors, as well as healthcare funding models. For every PET scanner available globally, there are four SPECT machines[4], making them a more accessible option when used with 99mTc – a radionuclide with a well-established supply chain, which can be produced in a bench-top generator.
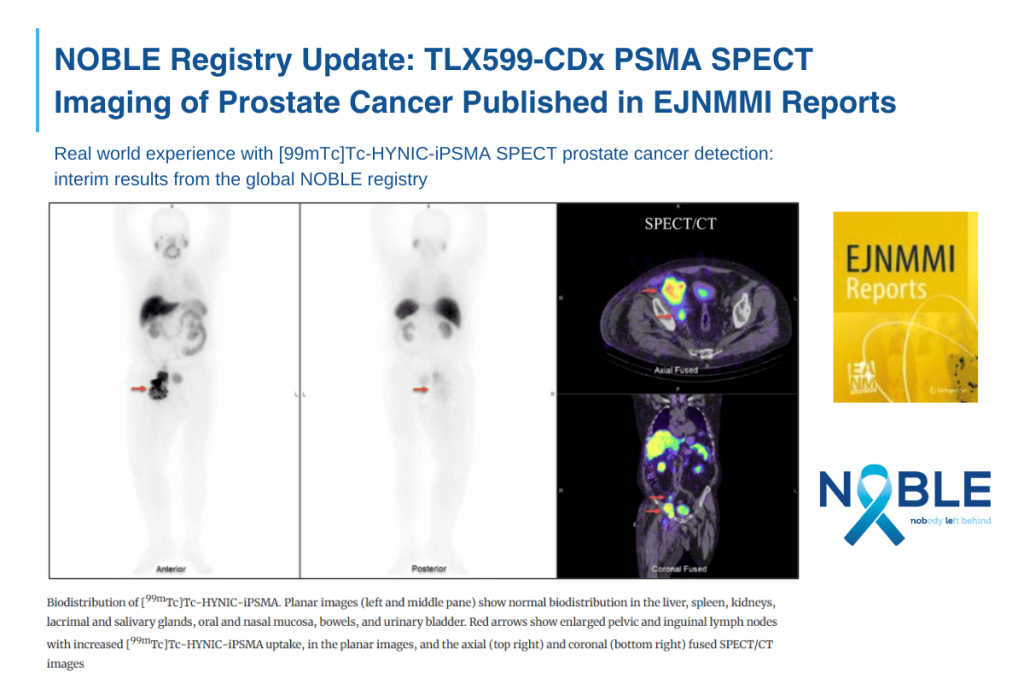
In the publication, the investigators report initial results based on a study of 40 patients in six countries[5], who received TLX599-CDx followed by planar and SPECT imaging. Investigators reported a change in management for 17 patients (42.5%) due to the use of SPECT-based PSMA imaging. No adverse events were reported. The authors conclude that technetium-based imaging “is a promising option to identify PSMA-positive prostate cancer on SPECT and could improve patient access to PSMA imaging worldwide”, fulfilling an unmet need for millions of patients with prostate cancer who do not have straightforward access to PET imaging.
Pete Tually, lead author, Director, TeleMed Remote Nuclear Medicine, and Principal Investigator for the Australian arm of NOBLE said, “The NOBLE Registry is an important initiative for increasing access to medicine globally, particularly for men in regional and remote locations. The interim results reported in our manuscript are promising, and we believe they provide a compelling basis for the further clinical study of technetium-99m-based PSMA imaging of prostate cancer.”
Rebecca Lo bue, CEO, Oncidium foundation, added, “PSMA-PET[6] imaging is today widely used in many countries worldwide, helping to extend life and improve treatment outcomes for men with prostate cancer through earlier diagnosis and better disease management. However, millions of men do not have access to this technology. These results from NOBLE represent an exciting milestone in bringing to market a powerful, cost-effective, and widely available alternative imaging tool that uses PSMA-SPECT technology.”
Dr. David N. Cade, Group Chief Medical Officer, Telix, continued, “Telix is proud to support the NOBLE Registry, which aims to improve access for patients who face geographic or economic barriers to state-of-the-art prostate cancer imaging. This publication by Pete Tually and his co-authors[7] signals a positive future for technetium-99m-based PSMA imaging, and we are excited to be part of this innovative program. As established leaders in PSMA imaging, Telix is committed to exploring multiple avenues to help men with prostate cancer get the diagnoses and treatments they need. We would like to thank all NOBLE investigators and the patients who have contributed towards this study.”
The EJNMMI Reports publication is available online at: https://link.springer.com/article/10.1186/s41824-024-00226-4
Telix and the Oncidium foundation are planning follow-on clinical activity for NOBLE. This may include collaboration with Rhine Pharma on expanded access or compassionate use programs, exploring the use of technetium-99m and rhenium-188 as a theranostic pair for prostate cancer. This initiative reflects Telix’s global commitment to access to medicine.
To read the full media release, click here
[1] Prostate-specific membrane antigen.
[2] Single photon emission computed tomography.
[3] Positron emission tomography/computed tomography.
[4] MEDraysintell.
[5] Australia, Azerbaijan, Egypt, Indonesia, Mexico, South Africa.
[6] Imaging of prostate-specific membrane antigen with positron emission tomography.
[7] Virginia Garcia Quinto (Hospital Galenia Department of Nuclear Medicine, Cancun, Mexico), Yehia Omar (Misr Radiology Center, Cairo, Egypt), Fuad Novruzov (Department of Nuclear Medicine, Azerbaijan National Centre of Oncology, Baku, Azerbaijan), Ryan Yudistiro (Department of Nuclear Medicine, Siloam Hospital, Jakarta, Indonesia), Mike Sathekge (University of Pretoria Nuclear Medicine Department, Gauteng, South Africa), Geoffrey Currie (School of Dentistry and Medical Sciences, Charles Sturt University, Bathurst, Australia), Paul Galette, Neel Patel, Tracey Brown and David Cade (Telix), Gabriel Bolland and Rebecca Lo bue (Oncidium foundation).
TLX599-CDx has not received a marketing authorisation in any jurisdiction.