X

Every man deserves access to adequate prostate cancer diagnostic imaging.
99mTc-iPSMA is authorised for investigational use only. None of the technologies described on the website have received a marketing authorisation in any jurisdiction.

Every man deserves access to adequate prostate cancer diagnostic imaging.


Every man deserves access to adequate prostate cancer diagnostic imaging.


Every man deserves access to adequate prostate cancer diagnostic imaging.
99mTc-iPSMA is authorised for investigational use only. None of the technologies described on the website have received a marketing authorisation in any jurisdiction.
99mTc-iPSMA is authorised for investigational use only. None of the technologies described on the website have received a marketing authorisation in any jurisdiction.

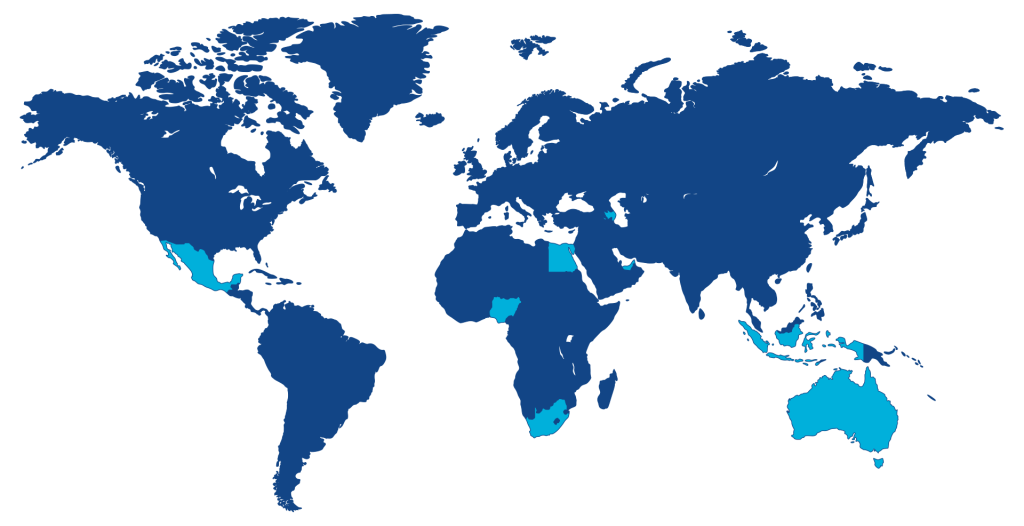
The NOBLE Registry is a collaboration combining the oncology and prostate cancer expertise of Oncidium foundation and Telix Pharmaceuticals. It is led by Principal Investigator Dr. Batool Albalooshi, Head of Nuclear Medicine and Molecular Imaging at the Dubai Health Authority, United Arab Emirates and supported by an expert global clinical leadership team.
The objective is to generate a data repository – which may inform further development of PSMA-SPECT products for emerging markets.
Ultimately, the Oncidium foundation and Telix aim to enable patients with prostate cancer, regardless of origin, technology availability or financial situation, to access PSMA-SPECT imaging for accurate diagnosis and treatment planning.
© Oncidium foundation 2021
This website is owned and maintained by the Oncidium foundation, with support from Telix Pharmaceuticals.
Prostate Cancer is the second most frequent cancer diagnosis in men and the fifth leading cause of cancer death worldwide [ref Globocan 2021]. Early detection can significantly reduce the mortality rate and in recent years much attention has focused on prostate-specific membrane antigen (PSMA) as a target for imaging and therapy using radionuclides for prostate cancer.
Screening for prostate cancer can start by taking a blood test to measure your PSA level. PSA (Prostate Specific Antigen) is a substance in the blood that is mainly made by the prostate gland. When higher-than-normal levels are noticed in men, it can be a sign of prostate conditions, including cancer.
To prepare for imaging to identify prostate cancer, a small amount of a radioactive substance can be injected into the patient’s body, which seeks out a marker expressed by cancer cells.
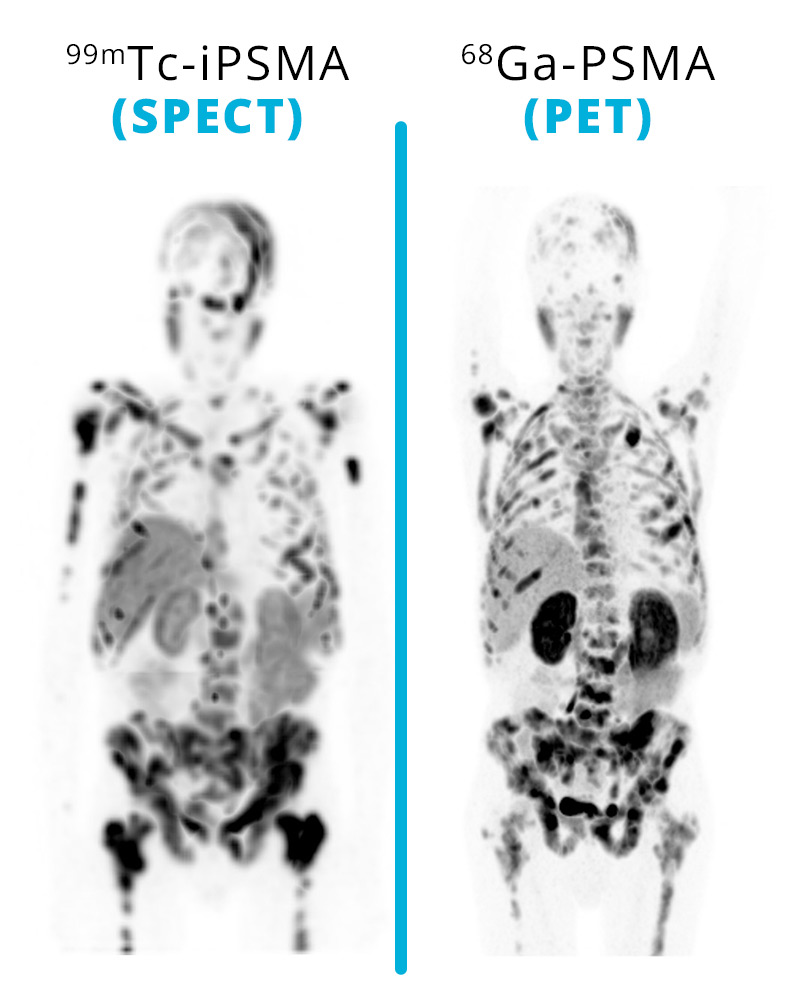
Globally, PSMA positron emission tomography (PSMA PET) is an emerging standard of care in prostate cancer imaging [ref: Hofman M, et al. The Lancet 2020; Trabulsi E, et al. Journal of Clinical Oncology 2020; National Comprehensive Cancer Network (NCCN) Guidelines 2021] with several products either approved or in development worldwide. Once approved these products will provide prostate cancer patients with access to PSMA-PET imaging technology, however PET technologies are scarce in some parts of the world and such imaging products may come at a high cost. The NOBLE registry is dedicated to developing an efficient and affordable alternative through SPECT-imaging, allowing many more patients to access a PSMA scan when indicated.
PSMA-SPECT products, when registered, could lead to a viable imaging tool for diagnosing prostate cancer in emerging countries, as an alternative to PSMA-PET.
SPECT cameras widely available in emerging countries
Identify and treat prostate cancer in men with advanced disease
Give access to an affordable and potentially effective diagnostic scan
Deliver publications and clinical practice Guidelines and Protocols
VS
SPECT and PET : MEDRaysintell report 2021, data 2020
Cancer Incidence : Globocan 2020
8 centers leading the investigation to provide real world evidence to deliver useful publications and clinical practice guidelines.









Principal investigator
Dr. Iván E. Díaz Meneses
Nuclear Medicine Physician and Professor







Implementation of Clinical Registry using 99mTc-iPSMA SPECT imaging for patients with prostate cancer.
Key clinical applications for PSMA SPECT:

Primary staging in newly diagnosed high-risk prostate cancer

Patient selection for PSMA targeted radio-ligand therapy (RLT)

Treatment planning for localized external-beam radiation (XRT)

Prostate imaging in patients with suspected biochemical recurrence (BCR)

Monitoring or response to systemic therapy
Lower cost
Gamma emitting radioisotope (tracer)
(e.g. 99mTc, 123I, 131I)
Higher cost
Positron emitting radioisotope (tracer)
(e.g. 18F, 68Ga)
(1) Courtesy: Dr. Ivan E. Diaz-Meneses (Nuclear Medicine Physician; MEXICO)
Single photon emission computed tomography (SPECT) and positron emission tomography (PET) are nuclear medicine imaging techniques which provide metabolic and functional information.
99mTc-iPSMA is authorised for investigational use only. None of the technologies described on the website have received a marketing authorisation in any jurisdiction.
Follow us to get real time results of the NOBLE Clinical Registry
(Press Releases, Latest News)
Proceeds will go to the Oncidium foundation and will be re-injected in the NOBLE Registry project.
The Oncidium foundation is a non-profit organization that focuses on promoting and supporting the development of Radiotheranostics by raising awareness and ultimately accelerating global access.
By supporting the NOBLE Registry project, you put your action at the heart of the fight against cancer!
The information on this Website is provided for information purposes only and made available on the understanding that it does not constitute professional or expert medical advice. If you are a patient using this Website, you should seek assistance from a health care professional when interpreting these materials and applying them to your individual circumstances.
If you have any concerns about your health, consult your general practitioner. Information provided on this Website does not imply endorsement of third-party services or products and cannot provide you with health and medical advice.
Use of site is subject to the terms of use and privacy policy.
If you choose not to accept the terms and conditions of use, the website page will close instantly.